










0 Reviews
2955 Views
1. Structure of Atoms 2. Classification of Elements and Periodocity of Properties 3. Chemical Bonding 4. Redox Reactions and Standard Electrode Potent .... Read More
Price: 799940
You Save 141 15% off
Price: 705940
You Save 235 25% off
*Buy both at: 1128 1880
You Save 752
40% off
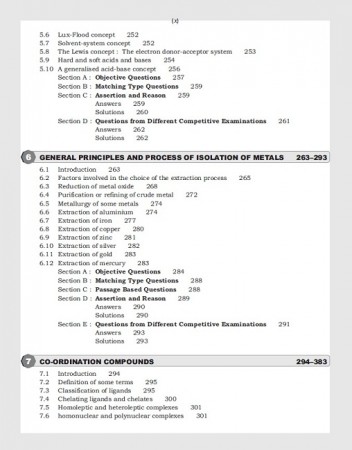
1. Structure of Atoms 2. Classification of Elements and Periodocity of Properties 3. Chemical Bonding 4. Redox Reactions and Standard Electrode Potential 5. Acids and Bases 6. General Principal and Process of Isolation of Metals 7. Co- Ordination Compounds 8. Hydrogen 9. The s- Block Elements 10. The p- Block Elements 11. d- and f- Block Elements 12. Sroichiometry and Quantitative Analysis 13. Qualitative Inorganic Analysis 14. Environmental Chemistry, Index
| Sr | Chapter Name | No Of Page |
|---|---|---|
| 1 | STRUCTURE OF ATOMS | 53 |
| 2 | CLASSIFICATION OF ELEMENTS AND PERIODICITY OF PROPERTIES | 44 |
| 3 | CHEMICAL BONDING | 100 |
| 4 | REDOX REACTIONS AND STANDARD ELECTRODE POTENTIAL | 47 |
| 5 | ACIDS AND BASES | 18 |
| 6 | GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF METALS | 31 |
| 7 | CO-ORDINATION COMPOUNDS | 90 |
| 8 | HYDROGEN | 27 |
| 9 | THE s-BLOCK ELEMENTS | 42 |
| 10 | THE p-BLOCK ELEMENTS | 108 |
| 11 | d- and f-BLOCK ELEMENTS | 60 |
| 12 | STOICHIOMETRY AND QUANTITATIVE ANALYSIS | 42 |
| 13 | QUALITATIVE INORGANIC ANALYSIS | 42 |
| 14 | ENVIRONMENTAL CHEMISTRY | 42 |